As news of yet another SARS-CoV-2 variant dominates headlines, the need for a truly comprehensive solution to identify and understand the rapidly mutating virus becomes even more important.
From Alpha, Beta, Delta to Omicron, scientists and health officials have been racing to keep up with emerging strains of the virus that have immobilized the world for nearly two years and caused nearly 5.5 million deaths.
The virus evolves to adapt to human physiology and evade immunity via mutations in its genetic sequence. Some of these mutations also disrupt the assays we use to detect and characterize SARS-CoV-2, so it is crucial to public health that we understand the implications of each new variant. Transmissibility, severity of illness, the efficacy of existing vaccines, and the development of new ones all rely on the ability to understand mutations as they arise.
Each mutation effects the virus in different ways. The N501Y mutation to the SARS-CoV-2 spike protein, for example, can cause the virus to bind better to human cells. The E484K mutation can potentially lessen the effectiveness of COVID-19 vaccines.
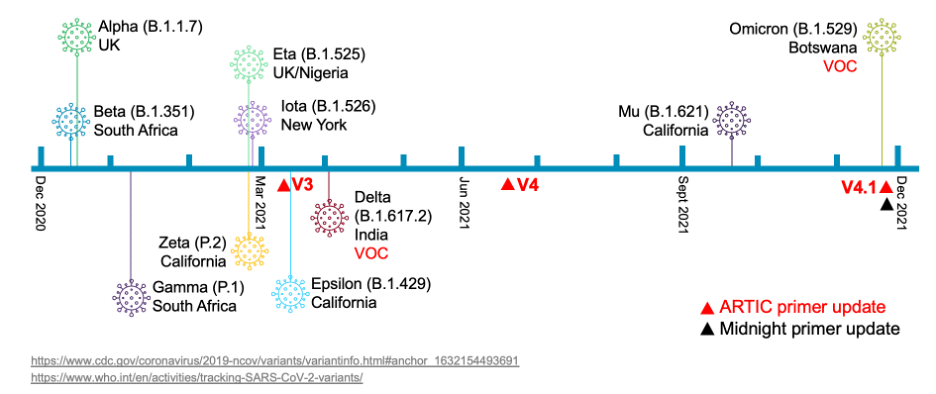
Still other mutations can affect the primer binding required for sequencing with PCR amplicon-based methods. These mutations have led to the need for periodic primer updates to keep pace with the evolving virus, and these updates can be slow to ship. The ARTIC V3 primers used in many commercial solutions, for instance, was superseded by the ARTIC V4 primer set in June 2021 to address mutations in the Beta (B.1.1.7) and Delta (B.1.617.2) strains, and 11 new primers were introduced five months later to address Omicron (B.1.1.529).
Here is a brief timeline of the emergence of different SARS-CoV-2 variant lineages, to date:
Simple, Swift Solution
Recently, South African scientists were able to overcome these challenges and successfully sequence the entire genome of the SARS-CoV-2 Omicron lineage with the PacBio HiFiViral SARS-CoV-2 Kit within mere weeks of the variant’s discovery.
The Inqaba Biotec team used the new PacBio HiFiViral kit to quickly capture the entire genome, delivering information that is crucial to public health.
“We were able to successfully sequence Omicron in our lab and reliably get complete coverage of the entire genome, including the highly mutated gene responsible for the spike protein,” said Hamilton Ganesan, Bioinformatics Manager at Inqaba Biotec. “It’s important to have this level of resolution to understand the full impact of this variant.”
The HiFiViral kit enabled rapid results—from RNA samples to analysis results in 28-42 hours, with 1-2.5 hours hands-on time—using a novel alternative to traditional PCR enrichment.
The alternative involves molecular inversion probes (MIPs), single-stranded DNA molecules containing two regions complementary to the target DNA. Because the process uses ~1,000 probes to tile the genome at 22-fold coverage (each base is covered by 22 probes), it has proven resilient to mutation-induced sequence dropouts.
To evaluate the performance of the HiFiViral kit against highly mutated variants, 44 likely Omicron samples with a broad range of Ct values were chosen based on amplicon dropout patterns in positive PCR tests.
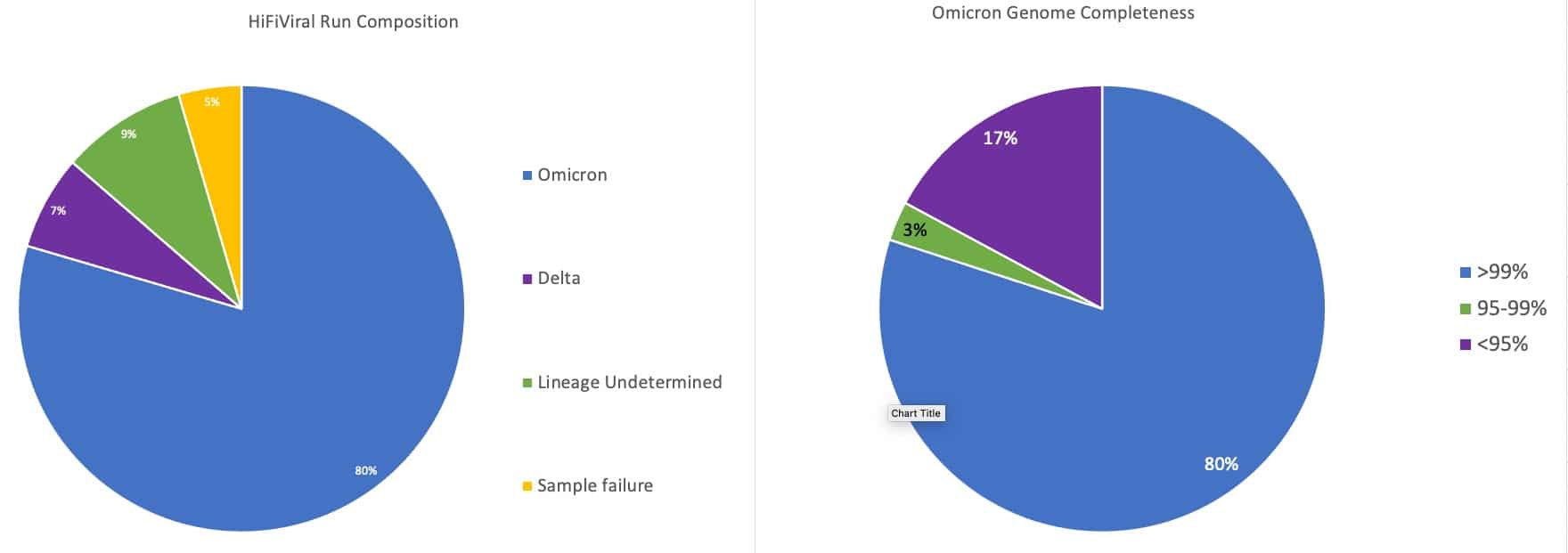
Of these, 38 samples could be assigned to a specific lineage, 35 of which were Omicron. As shown in Figure 1, the vast majority of the Omicron-identified samples had >99% genome completeness.
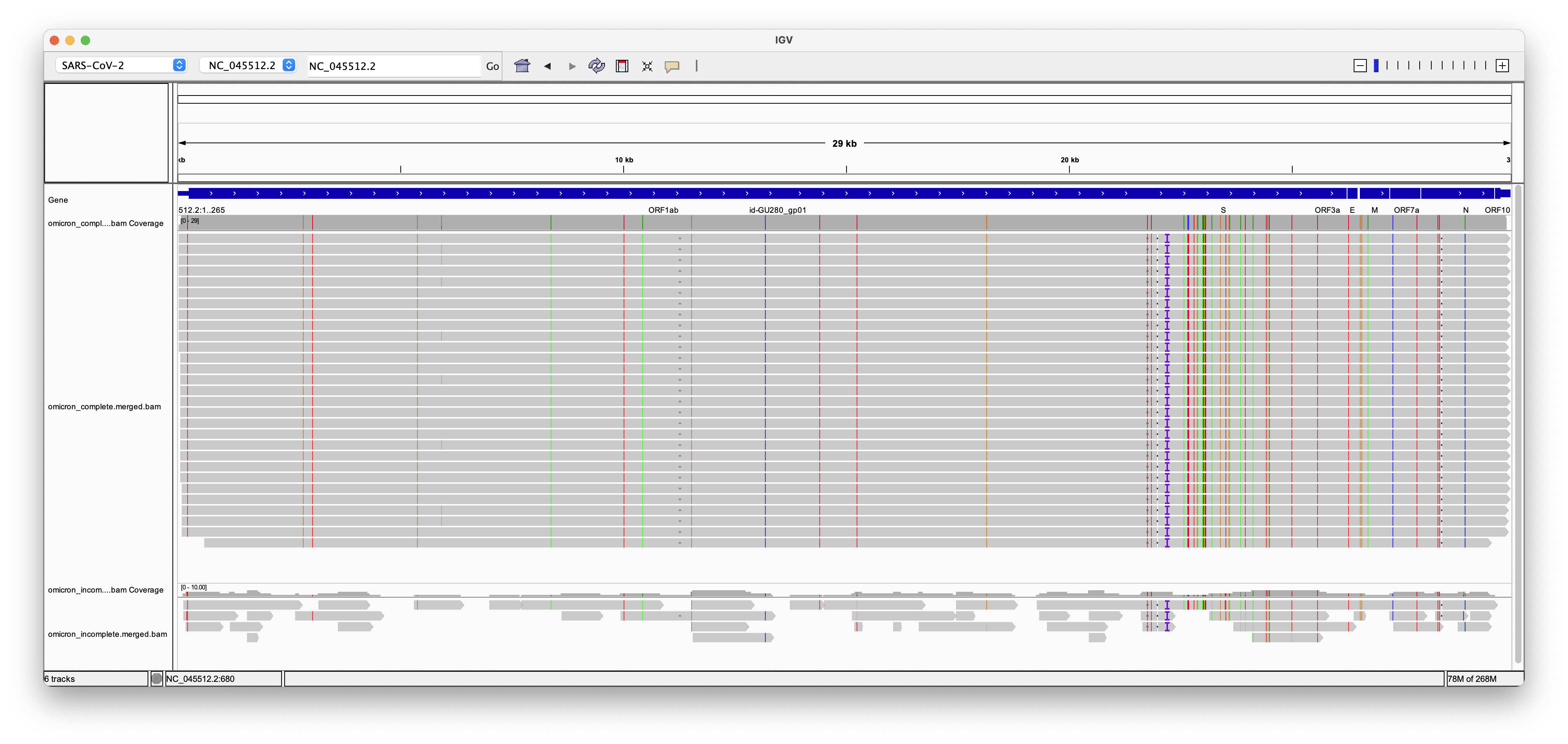
Aligning genome consensus sequences of complete and incomplete Omicron samples reveals that gaps in poorly covered samples do not show a consistent pattern, suggesting that coverage failures are a result of poor sample quality rather than a systematic failure due to mutations in the Omicron lineage (Figure 2).
Furthermore, the performance of the HiFiViral Kit matches the kit performance in previously common variants, including Delta and Alpha (Figure 3), demonstrating how this MIP-based method is a stable solution that does not require periodic updates and revalidation, despite the fast evolution of SARS-CoV-2.
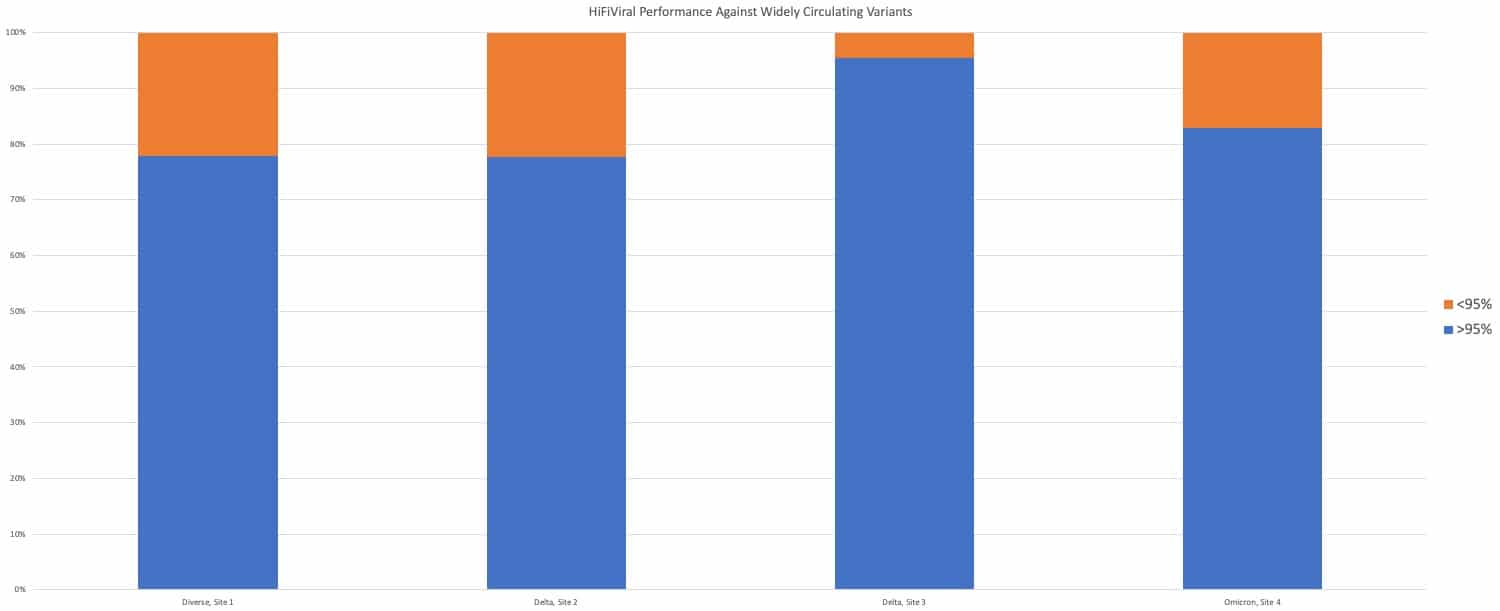 Figure 3. HiFiViral Kit data from four different sites shows consistent performance across runs that are composed of primarily Delta, primarily Omicron, or a mix of diverse lineages. From left to right, the runs had 96, 384, 896 or 44 samples. [/caption]
Figure 3. HiFiViral Kit data from four different sites shows consistent performance across runs that are composed of primarily Delta, primarily Omicron, or a mix of diverse lineages. From left to right, the runs had 96, 384, 896 or 44 samples. [/caption]
HiFi Data Advantages
As it turns out, combining MIPs with HiFi reads has several advantages for genomic surveillance beyond robust performance in diverse variants.
Because HiFi reads are long and accurate, fewer reads are required to obtain full genome coverage (~1,000 reads required using PacBio vs ~10,000 reads for 20x coverage using Oxford Nanopore or ~1,000,000 reads for 10x coverage using Illumina technology). Also, fewer reads means simpler analysis.
In addition to providing better data to researchers, the kit’s workflow requires less time, labor, and substantially fewer plastics and other consumables than other technologies – reducing potential supply chain bottlenecks that have challenged surveillance laboratories throughout the pandemic. The simplicity of the workflow, in particular, appealed to Ganesan.
“It allowed us to process, analyze, and turn around results in a rapid and efficient way that we couldn’t get from PCR-amplicon based methods,” he said.
With less pipetting, fewer plate transfers, and color-change indicators at every step, the workflow also reduces handling errors.
And, the kit provides exceptional flexibility. It allows labs to scale testing to meet different sample volumes and turnaround time needs. Using the Sequel IIe System, users can multiplex between 24 – 384 samples per SMRT Cell 8M or load up to 8 SMRT Cells to process up to 3,072 samples per week.
“This solution gives researchers around the globe a powerful tool to access complete genomic information, and we believe it will be essential in future-proofing labs against new variants,” said Christian Henry, Chief Executive Officer and President of PacBio. “Having a complete picture of the emerging variants will help public health entities better answer critical questions about how similar they are to other variants, their transmissibility, spread, implications for vaccine breakthrough, and potential occurrences of additional genome changes.”
We are very excited about the work Inqaba Biotec is doing to combat SARS-CoV-2 and even more excited about how our HiFiViral SARS-CoV-2 Kit is positioned to future-proof researchers against variants of all kinds.
Learn more about how PacBio technology can be harnessed in your COVID-19 research:
COVID-19 Resource page
SARS-CoV-2 Datasets
Resolving Viral Populations with HiFi
POSTER: HiFiViral SARS-CoV-2: A Kitted Solution for Genome Surveillance that is Robust Across Sample Input Quantities and New Variants
VIDEO: PacBio Sequencing for SARS-CoV-2 Surveillance
Introduction to HiFi Reads
Resources