Scientists at Yokohama City University Graduate School of Medicine and Osaka Women’s and Children’s Hospital have discovered a novel pathogenic variant associated with intellectual disability. They made the discovery using HiFi sequencing after previous short-read investigations failed to produce an answer.
In the journal Genomics, the team reports the case of 12-year-old monozygotic twin girls who exhibited developmental delays, severely drooping eyelids, and seizures since the age of 5 months. Clinical symptoms matched Dravet syndrome, but no molecular evidence was available to confirm that diagnosis. Their case had previously been analyzed with short-read exome sequencing, but no pathogenic variants were uncovered. Lead author Takeshi Mizuguchi, senior author Naomichi Matsumoto, and collaborators turned to HiFi sequencing and the Sequel II System “to search for variants that are unrecognized by exome sequencing,” they write.
While intellectual disability (ID) has been linked to variants in more than 500 genes, even the best analytical methods have a diagnostic success rate of less than 30%. “There are still many cases for which no molecular diagnosis has been possible,” the authors note. “Therefore, it is important to determine the molecular genetics of unsolved ID cases using new technologies.”
The scientists sequenced 15 kb size-selected libraries for one of the twins and both parents to generate highly accurate (>99% or Q20) long reads, known as HiFi reads. Next, the team used pbsv to call structural variants, and Google’s DeepVariant to call small variants and indels. This process highlighted hundreds of deletions, insertions, and duplications, plus seven inversions, in the twin’s genome that were potential de novo structural variants. “A 12-kb inversion disrupting the coding sequence of Bromodomain and PHD Finger containing 1 … immediately drew our attention,” the authors report, because variants in this region had been linked to an intellectual disability syndrome consistent with the twins’ symptoms. “Among the 16 possible de novo [structural variant] calls affecting RefSeq gene exons, no other genes were linked to an OMIM autosomal dominant disease entry,” they add.
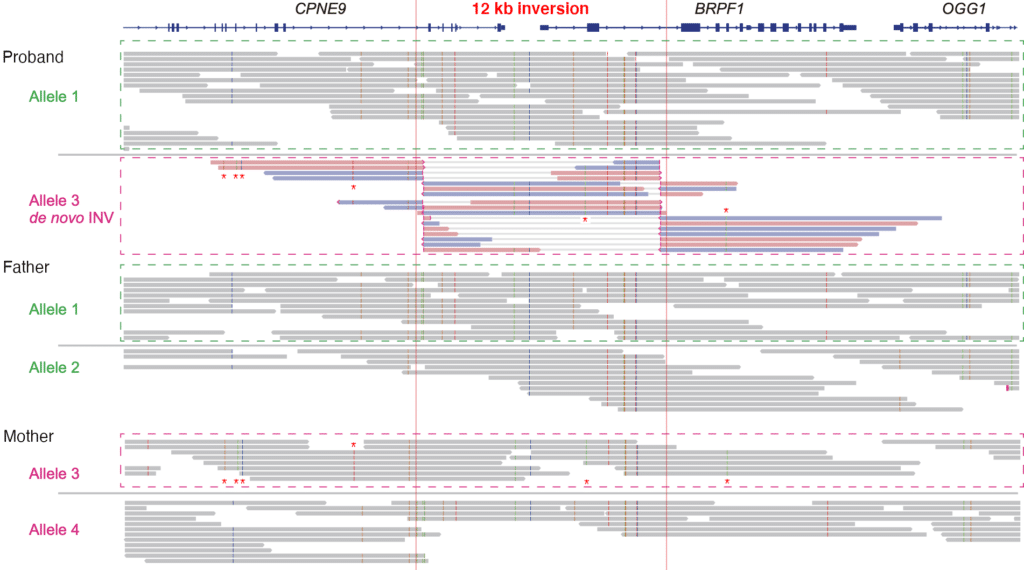
The 12 kb copy-neutral inversion was confirmed with Southern blot, which also showed that both parents and an unaffected older brother lacked the inversion. A breakpoint analysis found that “the two breakpoint junctions identified by Sanger sequencing and the pbsv inversion call were identical,” the team notes, “demonstrating the accuracy of HiFi long-read analysis.” The scientists also point out that not only was the inversion missed by exome sequencing due to its copy-neutral status and repetitive flanking sequence, but it also would have been missed by traditional chromosomal analysis, which has a lower limit of detection of 10 Mb. Finally, using the trio data with haplotype phasing, the team discovered that the inversion was a de novo variant on the maternally transmitted chromosome.
“Importantly, the current study demonstrates that inversions can now be accessed using an ‘unbiased-genomic’ strategy with no prior knowledge,” the authors write. “This state-of-the-art technology is advantageous for elucidating hitherto inaccessible genomic changes.”
To learn more, explore SMRT Sequencing workflows and additional resources on comprehensive variant detection and structural variant detection.
November 17, 2020 | Human genetics research